Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Gonzal z, M. A.*; Borodin, O.*; Kofu, Maiko; Shibata, Kaoru; Yamada, Takeshi*; Yamamuro, Osamu*; Xu, K.*; Price, D. L.*; Saboungi, M.-L.*
z, M. A.*; Borodin, O.*; Kofu, Maiko; Shibata, Kaoru; Yamada, Takeshi*; Yamamuro, Osamu*; Xu, K.*; Price, D. L.*; Saboungi, M.-L.*
Journal of Physical Chemistry Letters (Internet), 11(17), p.7279 - 7284, 2020/09
Times Cited Count:17 Percentile:78.45(Chemistry, Physical)Irisawa, Keita; Meguro, Yoshihiro
Journal of Nuclear Science and Technology, 54(3), p.365 - 372, 2017/03
Times Cited Count:2 Percentile:19.37(Nuclear Science & Technology)Yang, S.*; Katsumura, Yosuke*; Yamashita, Shinichi*; Matsuura, Chihiro*; Hiroishi, Daisuke*; Lertnaisat, P.*; Taguchi, Mitsumasa
Radiation Physics and Chemistry, 123, p.14 - 19, 2016/06
Times Cited Count:2 Percentile:19.57(Chemistry, Physical) -radiolysis of boiling water has been investigated. The G-value of H
-radiolysis of boiling water has been investigated. The G-value of H evolution was found to be very sensitive to the purity of water. In high-purity water, both H
evolution was found to be very sensitive to the purity of water. In high-purity water, both H and O
and O gases were formed in the stoichiometric ratio of 2:1; a negligible amount of H
gases were formed in the stoichiometric ratio of 2:1; a negligible amount of H O
O remained in the liquid phase. The G-values of H
remained in the liquid phase. The G-values of H and O
and O gas evolution depend on the dose rate: lower dose rates produce larger yields. To clarify the importance of the interface between liquid and gas phase for gas evolution, the gas evolution under Ar gas bubbling was measured. A large amount of H
gas evolution depend on the dose rate: lower dose rates produce larger yields. To clarify the importance of the interface between liquid and gas phase for gas evolution, the gas evolution under Ar gas bubbling was measured. A large amount of H was detected, similar to the radiolysis of boiling water. The evolution of gas was enhanced in a 0.5 M NaCl aqueous solution. Deterministic chemical kinetics simulations elucidated the mechanism of radiolysis in boiling water.
was detected, similar to the radiolysis of boiling water. The evolution of gas was enhanced in a 0.5 M NaCl aqueous solution. Deterministic chemical kinetics simulations elucidated the mechanism of radiolysis in boiling water.
Kozai, Naofumi; Onuki, Toshihiko; Komarneni, S.*
Journal of Materials Chemistry, 17(12), p.2993 - 2996, 2002/12
Here we report the extremely high and selective uptake of selenium oxyanions by a novel exchanger, Ni Zn
Zn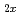 (OH)
(OH) (OCOCH
(OCOCH )
)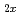 ・nH
・nH O that has brucite-type hydroxide layers with OCOCH
O that has brucite-type hydroxide layers with OCOCH anions in the interlayers. This Ni-Zn basic salt exhibited very high selectivity for Se(IV) (Kd = 9.0x10
anions in the interlayers. This Ni-Zn basic salt exhibited very high selectivity for Se(IV) (Kd = 9.0x10 ml/g with an initial Se(IV) concentration of 1x10
ml/g with an initial Se(IV) concentration of 1x10 M) in the presence of 0.1M Cl
M) in the presence of 0.1M Cl solution while the well known anionic clay, Mg
solution while the well known anionic clay, Mg Al(OH)
Al(OH) NO
NO ・nH
・nH O showed a Kd of 6.0x10
O showed a Kd of 6.0x10 ml/g under the same conditions. The uptake of Se(IV) on the Ni-Zn basic salt was found to be irreversible when treated with solutions containing 1N Cl
ml/g under the same conditions. The uptake of Se(IV) on the Ni-Zn basic salt was found to be irreversible when treated with solutions containing 1N Cl , 1N NO
, 1N NO
 , or 1N PO
, or 1N PO
 , while the Se(IV) sorbed on anionic clay was easily desorbed in a 1M Cl
, while the Se(IV) sorbed on anionic clay was easily desorbed in a 1M Cl solution. This novel exchanger also showed high Kd (2.6
solution. This novel exchanger also showed high Kd (2.6 10
10 ml/g at an initial Se concentration of 1
ml/g at an initial Se concentration of 1 10
10 M) for Se(VI) and therefore it is expected to be useful for decontamination and removal of selenium oxyanions from contaminated water.
M) for Se(VI) and therefore it is expected to be useful for decontamination and removal of selenium oxyanions from contaminated water.
;
Journal of Nuclear Materials, 140, p.32 - 43, 1986/00
Times Cited Count:14 Percentile:79.15(Materials Science, Multidisciplinary)no abstracts in English